
At HH Innovations, as one of the UK’s leading medical device prototyping companies, we understand that effective design, development, and manufacturing processes are essential for ensuring beneficial outcomes for patients requiring specialist medical care.
From medical technology used in intensive hospital wards, to prosthetics for daily use, medical devices increase the success rates of medical procedures and surgeries and improve the quality of life for patients with long-term health conditions.
In the medical device manufacturing industry, rigorous quality control and precision are key to ensuring high-quality medical technologies. These technologies support healthcare facilities to provide a high standard of care, and require rigorous testing and quality controls to ensure that they are fit for purpose.
We fully understand the responsibility involved in creating high-quality parts for the medical manufacturing industry, and feel privileged to support the healthcare workers operating on the frontline.
"Projects from concept through to finished supply of product"
We maintain a responsible controlled management theory, careful Quality Assurance and a professional engineering team in order to provide the best products and after-sales service to customers.
The design, development, prototyping, and manufacturing processes for medical devices are highly specialised, and an in-depth understanding of the medical industry is key to producing high-quality final products.
These processes involve several steps, including:

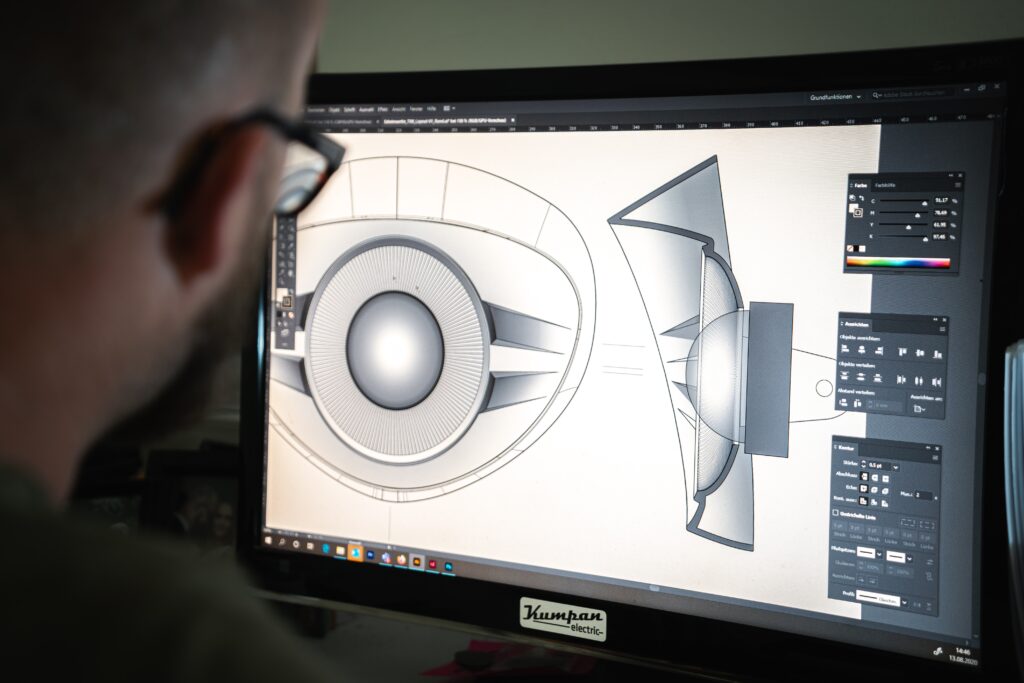

User-Centric Design
Clinicians and patients should be highly involved in the design process to ensure usability, ergonomics and safety.
This involves collaborating closely with specialists in the medical field, using focus groups of medical professionals and patients to ascertain their personal experiences of medical devices and what they require from a medical device, such as ergonomic design and pain reduction.
Both clinicians and patients should be involved in the design process to ensure that the product is not only usable and safe, but comfortable for patients.
At HH Innovations, we effectively utilise all the feedback given to us by our clients to ensure that clinician and patient experiences are held paramount throughout the design process. This ensures usable, safe, and ergonomic designs that promote a high standard of patient care in the healthcare sector.
Regulatory Compliance
At HH Innovations, we ensure that our designs meet regulatory standards and thoroughly document the process.
We are highly experienced at working to regulatory standards and always conduct thorough research, including consultations with professionals in the field to ensure that standards are upheld effectively throughout the design stage.
Innovation
Medical device development is constantly evolving, due to technological advancements in the medical field. These advanced technologies enable manufacturers to create high-quality medical devices that enhance the standard of care for patients and promote beneficial healthcare outcomes.
At HH Innovations, we are committed to using data-driven technologies to produce high-quality medical devices that meet the requirements of healthcare providers and the patients under their care.
Feasibility And Risk Assessment
At HH Innovations, we prioritise technical feasibility and thorough risk assessments to ensure that our designs are not only practically achievable – but most critically – safe for patients and medical staff.
We ensure that our designs are achievable within the designated timeline and budget, streamlining our processes to ensure that costs to medical providers are kept to a minimum.
Our risk assessment process allows us to identify and thus minimise any potential risks in advance of the prototyping stage, reducing costs and ensuring efficiency further down the line.
Interdisciplinary Collaboration
In the development stage, we continue to engage with medical specialists, implementing continuous feedback loops to ensure that the product is developed in line with their requirements. This close collaboration ensures that devices are technically sound, and successfully meet the needs of healthcare professionals and patients.
We also use continuous feedback loops to ensure that a variety of stakeholders are consulted throughout the design and development stages.
Regulatory Pathway Planning
At HH Innovations, we engage with regulatory bodies from the early stages of development, ensuring that our devices meet all relevant regulatory standards and requirements.
We plan and conduct clinical trials, ensuring that our designs are data-driven and successfully meet the needs of staff and patients. Throughout this process, we maintain clear and transparent communication with regulatory authorities.
This proactive approach ensures that the necessary approvals and certifications are achieved promptly. By prioritising regulatory pathway planning, we can ensure that our devices are market-ready for our clients and fully compliant with all industry regulations.
CNC machined prototypes will faithfully depict the aesthetics and features of the finished product, and are fully functional and ready for immediate use.



Prototyping is a crucial stage in the medical device development process. The prototyping process creates a prototype of the finished medical device, ensuring that it can be rigorously tested and checked to ensure that it is safe and fit for purpose before it progresses to the manufacturing stage.
Our prototyping process allows us to test the design of medical devices before production, enabling early identification and resolution of any potential flaws. We prioritise early-stage prototyping to prevent wasted resources and ensure a smoother development process.
By prototyping early, clients can make necessary adjustments before moving on to precision tooling, minimising the risk of financial losses. This approach leads to substantial cost savings and faster turnaround times, which is critical for fulfilling the requirements of the high-pressure medical industry.
At HH Innovations, we produce medical device prototypes using various processes such as vacuum casting, fabrication, SLS, SLA, FDM, and CNC prototype machining.
Scalability And Efficiency
Scalability and efficiency are critical considerations throughout our manufacturing process. We streamline production using advanced automation techniques to scale up manufacturing without compromising the quality of our products.
This approach enables us to ensure the process is scalable for growth while maintaining cost-effectiveness and consistency in our products.
Quality Control
In the quality control phase, we ensure that thorough testing has been conducted at every stage of the manufacturing process, meeting the requirements of stringent regulatory standards. By ensuring strict compliance with regulatory standards, we can promote the safety, effectiveness, and reliability of our finished medical devices.
Our quality control protocols include regular inspections, rigorous testing, and validation processes to identify and resolve any potential issues before the device is released to market.
Manufacturing
At HH Innovations, we are proud to partner with HH Plastics, plastic injection moulding specialists with extensive production facilities based in the economic centres of Shenzhen and Dongguan, China.
This enables us to give our clients full access to their team of highly experienced manufacturing specialists and manufacturing facilities spanning over 10,000 square metres.
Offering subcontract sourcing, assembly, and finished product supply services, we can guarantee that each finished product meets our clients’ product specifications and quality standards.


CNC Prototyping For Medical Devices
CNC machining allows us to create prototypes using materials that closely match the final product. This means the prototypes will have the same or similar colours, textures, and properties as the finished device, providing more reliable testing results.
This is essential in the medical industry to ensure that designs meet all the necessary specifications – like for example, by ensuring that the prototype can be made available in a range of skin tones for prosthetics.
Our CNC machined prototypes are true reflections of the finished design. They are immediately suitable for rigorous medical testing without any further adjustments or enhancements.
CNC machining allows us to create prototypes using materials that closely match the final product. This means the prototypes will have the same or similar colours, textures, and properties as the finished device, providing more reliable testing results.
This is essential in the medical industry to ensure that designs meet all the necessary specifications – like for example, by ensuring that the prototype can be made available in a range of skin tones for prosthetics.
Our CNC machined prototypes are true reflections of the finished design. They are immediately suitable for rigorous medical testing without any further adjustments or enhancements.
At HH Innovations, we are proud to maintain rigorous Quality Assurance and Quality Control measures throughout our design, development, prototyping, and manufacturing processes. We have personally developed our Quality Assurance and Quality Control measures according to our extensive expertise in the product development industry.
Our focus on achieving high-quality products, using principles of precision and process management, has enabled us to ensure that each of our products performs exceptionally on application.
At HH Innovations, we are proud to hold ISO 9001:2015 Certification for the Design and Manufacture of Plastic Mould And Accessories. We are committed to ensuring that we continue to meet these standards, by upholding rigorous Quality Assurance and Quality Control measures throughout our operations.
At HH Innovations, we are proud to maintain rigorous Quality Assurance and Quality Control measures throughout our design, development, prototyping, and manufacturing processes. We have personally developed our Quality Assurance and Quality Control measures according to our extensive expertise in the product development industry.
Our focus on achieving high-quality products, using principles of precision and process management, has enabled us to ensure that each of our products performs exceptionally on application.
At HH Innovations, we are proud to hold ISO 9001:2015 Certification for the Design and Manufacture of Plastic Mould And Accessories. We are committed to ensuring that we continue to meet these standards, by upholding rigorous Quality Assurance and Quality Control measures throughout our operations.
At HH Innovations, we take pride in being part of the HH Group, established in 2013. Our team of experts specialises in product design, manufacturing, and injection moulding processes, harnessing cutting-edge design and production facilities to create premium-quality products for our global clients.
Our headquarters are based in the UK under the leadership of our experienced management team, with manufacturing facilities based in the economic hubs of Shenzhen and Dongguan, China.
We excel in delivering high-quality design and production services, with a strong focus on catering to blue-chip companies across diverse industries such as the Medical, Consumer Electronics, Home Appliances, and Automotive sectors.
At HH Innovations, we take pride in being part of the HH Group, established in 2013. Our team of experts specialises in product design, manufacturing, and injection moulding processes, harnessing cutting-edge design and production facilities to create premium-quality products for our global clients.
Our headquarters are based in the UK under the leadership of our experienced management team, with manufacturing facilities based in the economic hubs of Shenzhen and Dongguan, China.
We excel in delivering high-quality design and production services, with a strong focus on catering to blue-chip companies across diverse industries such as the Medical, Consumer Electronics, Home Appliances, and Automotive sectors.



| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |